Ammonia production via the Haber-Bosch process is one of the most energy-intensive and thermodynamically delicate chemical reactions in industrial chemistry. Despite advances in catalyst technology and process control, underperformance in an ammonia synthesis tower or column often results from a lack of balance between reaction kinetics and heat management. If the reaction proceeds too slowly, conversion efficiency drops. If temperatures aren’t properly controlled, equilibrium shifts unfavorably or catalysts degrade. Mismanaging either aspect causes high energy consumption, catalyst poisoning, reduced ammonia yield, and dangerous thermal runaways.

Reaction kinetics and heat management critically influence ammonia synthesis tower performance by determining reaction rates, equilibrium conversion, and catalyst activity. The exothermic nature of the N₂ + 3H₂ ⇌ 2NH₃ reaction demands precise thermal control: while higher temperatures accelerate kinetics, they shift equilibrium away from ammonia formation. Efficient heat recovery, inter-stage cooling, and optimized catalyst bed design are essential to maintain both reaction progression and safe operation.
This balance between kinetics and thermodynamics governs how much ammonia is produced per pass, how often catalyst beds must be replaced, and how efficiently the tower converts raw materials into product. Understanding the interplay of these factors is essential to unlock maximum yield, minimize energy use, and ensure long-term process stability.
Higher temperatures always increase ammonia yield in the synthesis tower.False
Although higher temperatures speed up reaction rates, they shift the equilibrium toward reactants in the exothermic ammonia synthesis, reducing final yield.
Ammonia synthesis is an exothermic reaction, which complicates heat management in the reactor column.True
The exothermic nature of ammonia synthesis releases significant heat, requiring controlled cooling between catalyst beds to avoid deactivating the catalyst or reversing the reaction.
Reaction Kinetics – The Foundation of Ammonia Synthesis
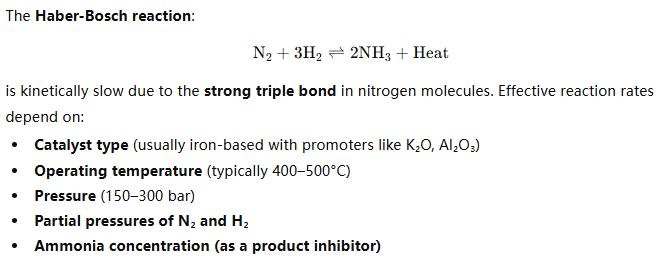
Even with optimal conditions, per-pass conversion rarely exceeds 20–25% due to equilibrium constraints, necessitating recycle loops.
Key Kinetic Constraints:
| Factor | Effect on Reaction Rate |
|---|---|
| Temperature ↑ | Rate ↑ (Arrhenius law), but equilibrium ↓ |
| Pressure ↑ | Rate and yield ↑ (Le Chatelier’s principle) |
| Catalyst surface area ↑ | Rate ↑ (more active sites) |
| Ammonia concentration ↑ | Rate ↓ (product inhibition) |
Modern ammonia towers utilize multiple catalyst beds with inter-stage cooling to maintain favorable conditions throughout the tower, enhancing kinetic performance without thermodynamic penalty.
Heat Management – Controlling an Exothermic Reaction
The ammonia synthesis reaction releases large amounts of heat (ΔH = –92.4 kJ/mol). Left uncontrolled, this can cause:
- Temperature runaway, damaging catalyst
- Equilibrium reversal, decreasing yield
- Non-uniform catalyst activity, reducing bed life
Strategies for Effective Heat Management:
- Inter-stage Cooling
- Between catalyst beds, heat exchangers lower gas temperature to optimal kinetic/equilibrium balance.
- Enables multi-bed operation with partial equilibrium conversion after each cooling stage.
- Heat Recovery Systems
- Hot reaction gases transfer heat to incoming feed gases, improving energy efficiency.
- Often implemented via waste heat boilers or economizers.
- Internal Heat Exchangers
- Embedded within the synthesis column for rapid temperature control.
- Improves temperature uniformity across catalyst beds.
- Quenching
- Injection of cold feed gas or recycled gas to cool reaction mixture dynamically.
- Fast but harder to control precisely compared to heat exchangers.
Here’s a conceptual thermal profile of a typical three-bed ammonia synthesis tower:
| Catalyst Bed | Inlet Temp (°C) | Outlet Temp (°C) | Heat Removal Method |
|---|---|---|---|
| Bed 1 | 400 | 500 | Inter-bed exchanger |
| Bed 2 | 400 | 480 | Recycle gas quench |
| Bed 3 | 390 | 450 | Final product cooling |
Interaction of Kinetics and Thermodynamics – The Balancing Act
One of the most challenging aspects of ammonia synthesis is the contradiction between optimal kinetic and thermodynamic conditions:
| Variable | Favors Reaction Rate (Kinetics) | Favors High Yield (Equilibrium) |
|---|---|---|
| High Temperature | ✅ Faster reaction | ❌ Lower equilibrium yield |
| High Pressure | ✅ Increases collision frequency | ✅ Drives equilibrium to product |
Therefore, reactor columns are designed with compromise zones:
- Temperature sweet spots (~430–450°C) balance rate and yield.
- Multi-bed configurations allow the reaction to proceed incrementally without extreme temperature rise.
- Recycle systems recover unreacted gases for another pass.
Let’s model this with performance simulation data:
| Parameter | Case A (Uncontrolled Heat) | Case B (Inter-cooled Beds) |
|---|---|---|
| Reactor Peak Temp | 540°C | 480°C |
| Per-Pass Yield | 16% | 23% |
| Catalyst Life | 2 years | 5 years |
| Ammonia Loss | High (equilibrium reversed) | Minimal |
Column Design Considerations for Optimizing Both Kinetics and Heat
An ammonia synthesis tower must be precisely engineered for gas distribution, pressure stability, and thermal management. Key components include:
- Catalyst support grids: Ensure even flow through catalyst beds.
- Inter-bed heat exchangers: Often shell-and-tube or hairpin types designed for high pressure and gas compatibility.
- Gas distributors: Maintain radial and axial flow uniformity to avoid channeling.
- Thermowells and sampling ports: Enable real-time temperature tracking and control.
Here’s an overview of critical design parameters:
| Design Feature | Purpose |
|---|---|
| Multi-bed configuration | Prevents thermal runaway, increases yield |
| High-pressure shell (up to 300 bar) | Maintains high collision frequency for kinetics |
| Radial gas flow | Enhances contact with catalyst surface |
| Thermal insulation | Retains energy, prevents heat loss |
| Alloy construction (e.g., 25Cr-20Ni steel) | Resists hydrogen embrittlement and corrosion |
Real-World Case: Ammonia Plant Upgrade with Enhanced Heat Management
Plant: Ammonia production facility, Middle East
Issue: Low ammonia yield (17%) and catalyst failure after 18 months
Analysis: Excessive internal temperatures due to lack of intermediate cooling
Solution:
- Added two inter-bed exchangers
- Installed real-time temperature sensors and automated control loop
- Switched to lower activation energy catalyst
Result:
| Metric | Before Upgrade | After Upgrade |
|---|---|---|
| Peak Reactor Temp | 540°C | 470°C |
| Per-Pass Conversion | 17% | 24% |
| Catalyst Life | 18 months | 48 months |
| Energy Consumption | 10.2 Gcal/ton NH₃ | 8.4 Gcal/ton NH₃ |
This case underscores the critical importance of integrating heat management with kinetic control in ammonia synthesis design.
Conclusion
Efficient ammonia synthesis requires simultaneous control of reaction kinetics and heat management. While high temperatures accelerate the reaction, they hurt equilibrium; while cooling preserves yield, it slows the process. Mastering this balance through smart column design, multi-bed staging, and precise thermal control is what separates high-performing synthesis systems from inefficient ones. Without these controls, even the best catalyst won’t deliver the expected performance or lifespan.
Optimize Your Ammonia Synthesis Reactor Today
Looking to boost yield, reduce catalyst costs, or optimize energy consumption in your ammonia synthesis tower or column? Contact our engineering team for a custom simulation, redesign consultation, or heat exchanger integration plan. Let’s engineer your system for peak performance and long-term reliability.
References
- Ammonia Synthesis | Thermodynamics and Kinetics – https://www.chemguide.co.uk/physical/equilibria/haber.html – ChemGuide
- Heat Management in Ammonia Reactors – https://www.sciencedirect.com/science/article/pii/S1876610217311472 – ScienceDirect
- The Haber-Bosch Process – https://pubs.acs.org/doi/10.1021/acs.jchemed.6b00227 – ACS Publications
- Ammonia Production Technologies – https://www.encyclopedia.com/science/news-wires-white-papers-and-books/ammonia-production – Encyclopedia.com
- Reactor Design for Ammonia Synthesis – https://www.engineeringtoolbox.com/ammonia-synthesis-d_2034.html – Engineering Toolbox
- Kinetic Models for Ammonia Synthesis – https://www.researchgate.net/publication/325430725 – ResearchGate












