Send Email
Call / WhatsApp Anytime
Send Email
Call / WhatsApp Anytime
A catalytic reactor is a type of pressure vessel specifically designed to facilitate heterogeneous catalytic reactions, in which reactants flow over or through solid catalyst beds. These reactors are widely used in oil refining, petrochemicals, fertilizer production, and synthetic gas processing, where they enable selective conversion of feedstocks under precise temperature and pressure conditions. Catalytic reactors are typically fixed-bed, multi-stage, high-pressure vessels, engineered for long-term, continuous operation.
Weihai Shidao Heavy Industry (WSHI) specializes in the design and fabrication of high-performance industrial reactors for complex chemical, petrochemical, pharmaceutical, and energy applications. Our reactor vessels are built to endure extreme pressure, temperature, and corrosive conditions, providing stable reaction environments for continuous and batch processing.













| Classification | Type | Description | Typical Applications |
|---|---|---|---|
| By Catalyst Phase | Heterogeneous Catalytic Reactor | Catalyst is in solid phase; reactants are gas or liquid | Hydrocracking, catalytic reforming, ammonia synthesis |
| Homogeneous Catalytic Reactor | Catalyst and reactants are in the same phase (typically liquid) | Esterification, fine chemical synthesis | |
| By Flow Pattern | Plug Flow Reactor (PFR) | Reactants flow in one direction with minimal mixing | Continuous processing with high selectivity |
| Backmixed Reactor (CSTR) | Reactants are well-mixed throughout the reactor volume | Liquid-phase batch reactions, homogeneous catalysis | |
| By Catalyst Bed Type | Fixed-Bed Reactor | Catalyst remains static; flow passes through the packed bed | Hydrotreating, reforming, methanol synthesis |
| Moving-Bed Reactor | Catalyst flows slowly and may be regenerated externally | Coal liquefaction, heavy oil upgrading | |
| Fluidized-Bed Reactor | Catalyst particles are suspended in gas flow | Fluid catalytic cracking (FCC), partial oxidation | |
| Trickle-Bed Reactor | Liquid trickles through packed catalyst; gas/liquid/solid contact | Diesel hydrotreating, hydrodesulfurization (HDS) | |
| By Operation Mode | Continuous Catalytic Reactor | Continuous feed and discharge of fluids and product | Large-scale petrochemical or fuel upgrading units |
| Batch Catalytic Reactor | Reactants processed in batches with full mixing and controlled reaction time | Specialty chemicals, pharmaceutical synthesis |
Certified Safety, Reliable Quality, Custom-Engineered Pressure Vessels.
— Globally Compliant, Built for Harsh Industrial Conditions.
Catalytic reactors play a critical role in modern industrial processes where high-efficiency chemical conversion, selective reaction control, and long catalyst life are essential. These reactors are engineered to facilitate gas-solid, liquid-solid, or gas-liquid-solid catalytic reactions under controlled pressure and temperature, and are widely deployed across refining, petrochemicals, chemical synthesis, energy, and environmental sectors.
Oil Refining
Catalytic reactors are extensively used in hydrocracking, hydrotreating, and catalytic reforming units. These fixed-bed, high-pressure reactors process heavy hydrocarbons into lighter, cleaner fuels such as diesel, gasoline, and naphtha while removing sulfur, nitrogen, and aromatics to meet ultra-low emission standards.
Petrochemicals
In the production of methanol, formaldehyde, and olefins, catalytic reactors enable precise control of reaction kinetics and catalyst activity. Examples include methanol synthesis reactors using Cu/ZnO catalysts and alkylation units utilizing solid acid catalysts under mild to moderate conditions.
Fertilizer & Syngas Industry
Catalytic reactors are central to ammonia synthesis, urea production, and syngas generation. Ammonia converters operate at extremely high pressures and temperatures using multi-bed iron-based catalysts, while methanation and water-gas shift reactors optimize syngas composition for downstream conversion.
Chemical & Fine Chemicals
In fine chemical and specialty material production, batch or continuous catalytic reactors support esterification, oxidation, hydrogenation, and polymerization processes. These systems often use homogeneous or immobilized catalysts and require high product selectivity and flexible reaction control.
Hydrogen Production & Energy
Catalytic steam reforming and partial oxidation reactors are used in hydrogen plants to convert hydrocarbons into hydrogen-rich synthesis gas. These reactors must withstand high thermal stress and enable efficient heat integration to support sustainable hydrogen generation.
Environmental Applications
Catalytic reactors are also applied in VOC abatement, catalytic incineration, selective catalytic reduction (SCR), and gas purification systems. These units help remove pollutants from flue gas and industrial exhaust using noble metal or vanadium-based catalysts.
Catalytic reactors are specialized vessels designed to carry out chemical reactions in the presence of a catalyst, typically under controlled pressure and temperature conditions. Their primary function is to accelerate reaction rates, improve selectivity, and reduce energy requirements in industrial processes without the catalyst itself being consumed.
Facilitate Chemical Conversion
They enable the transformation of raw materials (e.g., hydrocarbons, syngas, alcohols) into more valuable products (e.g., fuels, chemicals, fertilizers) through catalytic pathways.
Enhance Reaction Efficiency
Catalysts lower the activation energy of reactions, allowing processes to occur faster and more completely at lower temperatures or pressures than non-catalytic systems.
Improve Product Selectivity
Catalytic reactors help guide reactions toward desired products while minimizing unwanted byproducts, especially important in fuel refining, polymerization, and fine chemical production.
Enable Continuous Industrial Processes
Most catalytic reactors are built for long-term, continuous operation in fixed-bed, fluidized-bed, or trickle-bed configurations to support 24/7 production in refineries and chemical plants.
Support Environmental & Energy Goals
Catalytic reactors are used in clean energy (e.g., hydrogen production), emissions control (e.g., SCR systems), and gas purification processes to reduce carbon footprint and comply with environmental regulations.
Catalytic reactors provide the controlled environment and flow configuration necessary for catalyst-based reactions to proceed efficiently, safely, and at industrial scale. They are essential to the global production of fuels, chemicals, plastics, fertilizers, and clean energy.
One of the most widely used and technically significant examples of a catalytic reactor is the Hydrocracking Reactor in oil refineries.
Function:
A hydrocracking reactor is a high-pressure, fixed-bed catalytic reactor that converts heavy hydrocarbons (like vacuum gas oil or atmospheric residue) into lighter, more valuable products such as diesel, jet fuel, and naphtha, using hydrogen gas and solid catalysts.
How it works:
Heavy hydrocarbon feedstock is mixed with hydrogen and passed through one or more beds of bifunctional catalysts (typically metal + acidic support). The reaction occurs at high temperature (350–450°C) and high pressure (100–200 bar). The catalysts promote both cracking of large hydrocarbon molecules and hydrogenation of the resulting fragments, producing cleaner, low-sulfur fuels.
Reactor Type:
Fixed-bed catalytic reactor
Multi-bed configuration with inter-bed quench zones
Designed for continuous operation
Constructed to ASME/PED pressure vessel codes
Applications:
Diesel upgrading
ULSD (ultra-low sulfur diesel) production
Residuum conversion in complex refineries
| Reactor Type | Application |
|---|---|
| Ammonia Converter | Catalytic synthesis of NH₃ from H₂ and N₂ |
| Methanol Synthesis Reactor | CO + H₂ → CH₃OH |
| FCC Reactor | Fluid catalytic cracking of heavy oil |
| SCR Reactor | NOx reduction in flue gas treatment |
| Hydrogenation Reactor | Selective hydrogenation of olefins or aromatics |
Yes, a catalytic reactor is essential in the industrial production of formaldehyde, particularly through the oxidation of methanol.
In formaldehyde production, methanol (CH₃OH) is partially oxidized in the presence of oxygen or air over a solid catalyst inside a fixed-bed catalytic reactor.
CH3OH+12O2→HCHO+H2O\text{CH}_3\text{OH} + \frac{1}{2} \text{O}_2 \rightarrow \text{HCHO} + \text{H}_2\text{O}
Catalyst:
Silver-based (Ag) catalysts for high-conversion, high-temperature processes
Iron-molybdenum (Fe-Mo) oxide catalysts for lower temperature operation
Reactor Type:
Fixed-bed catalytic reactor, often multi-tubular, with heat exchangers for temperature control
Operates at 250–650°C depending on catalyst
Typically includes heat recovery systems for energy efficiency
Converts methanol into formaldehyde efficiently and selectively
Maintains precise temperature control to avoid over-oxidation (which would produce CO₂)
Designed for continuous operation, often in large-scale chemical complexes
Can integrate heat recovery for steam production (used in downstream urea-formaldehyde or phenolic resin synthesis)
A catalytic reactor is a core component of formaldehyde production plants. It enables the efficient and selective oxidation of methanol using solid catalysts in a fixed-bed design, supporting large-scale, continuous manufacturing.
Would you like a diagram of this process or a formaldehyde catalytic reactor product brief?
A catalytic reactor works by providing a controlled environment where chemical reactions are accelerated by a catalyst. The catalyst is not consumed during the reaction but facilitates the transformation of reactants into products more efficiently—typically at lower temperatures and pressures than would otherwise be required.
Catalytic reactors rely on heterogeneous catalysis in most industrial cases, where:
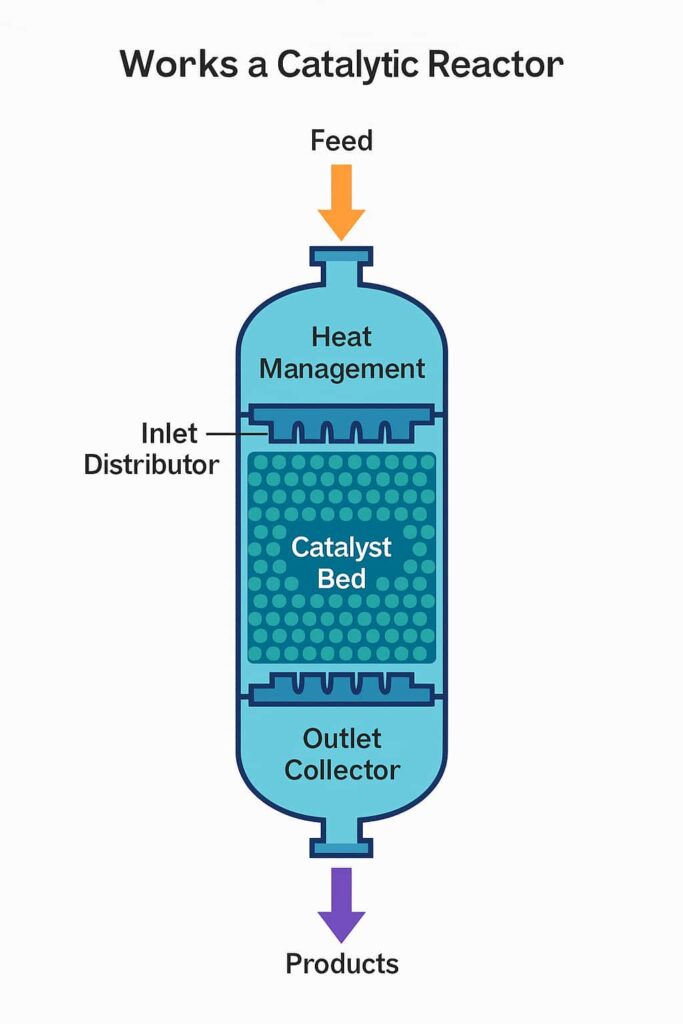
| Component | Function |
|---|---|
| Catalyst Bed | Provides reactive surface area |
| Inlet Distributor | Ensures uniform feed distribution |
| Quench System | Controls temperature between catalyst beds |
| Outlet Collector | Captures product flow evenly |
| Shell & Internals | Withstand pressure, thermal load, and corrosion |
A catalytic reactor enables fast, selective, and energy-efficient chemical reactions by bringing reactants into intimate contact with a catalyst under optimized flow, pressure, and temperature. It is a core unit in many processes across refining, petrochemicals, energy, and environmental applications.
Both catalytic converters and thermal reactors are used to reduce harmful emissions in exhaust gases, but they work by different mechanisms, have different operating conditions, and are applied in different contexts.
| Aspect | Catalytic Converter | Thermal Reactor |
|---|---|---|
| Working Principle | Uses a catalyst (typically platinum, palladium, rhodium) to accelerate chemical reactions | Relies on high temperature alone to oxidize unburned hydrocarbons and CO |
| Reaction Type | Catalytic oxidation/reduction (e.g., CO to CO₂, NOx to N₂) | Thermal oxidation without a catalyst |
| Operating Temperature | Works best between 250°C–600°C | Requires much higher temperatures, typically >800°C |
| Efficiency at Low Temp | High – begins working once catalyst lights off (~250°C) | Low – less effective at low to medium temperatures |
| Emission Control | Reduces CO, NOx, and hydrocarbons (HCs) | Mainly reduces CO and hydrocarbons, not NOx |
| Applications | Widely used in modern internal combustion engines, especially cars and trucks | Older or low-cost exhaust systems (e.g., 1970s emission control in motorcycles, aircraft) |
| Complexity & Cost | More complex and expensive (due to noble metals) | Simpler, lower cost, but less efficient |
| Maintenance | Catalyst can degrade or poison over time | Less maintenance-sensitive but less controllable |
A catalytic converter is more efficient, especially at lower temperatures, and is the standard solution in modern automotive emissions systems.
A thermal reactor is a simpler device that relies on high heat to burn off pollutants but is less effective and largely obsolete in modern systems.
The price of a catalytic reactor can vary widely depending on factors such as reactor size, pressure rating, materials of construction, catalyst type, process application, and customization requirements. Below is a professional breakdown:
| Reactor Type | Approximate Price Range |
|---|---|
| Lab-scale catalytic reactor | $5,000 – $30,000 |
| Pilot-scale catalytic reactor | $30,000 – $150,000 |
| Fixed-bed industrial reactor | $100,000 – $1,000,000+ |
| Fluidized-bed catalytic reactor | $300,000 – $2,000,000+ |
| High-pressure hydrocracking reactor | $500,000 – $5,000,000+ |
Note: These prices are indicative and may vary significantly based on project scope, pressure/temperature ratings (e.g., ASME/PED), alloy selection (e.g., SS304, SS316, Inconel), and catalyst system integration.
For an accurate price, manufacturers typically require details such as:
Would you like a pricing request template (RFQ) or a catalog layout for catalytic reactors by type and cost range?
Looking for High-Performance Industrial Reactors?
WSHI delivers custom-engineered reactor pressure vessels designed to meet the strictest demands of modern chemical and energy industries. Whether you need a high-pressure hydrogenation reactor, a corrosion-resistant lined vessel, or a precision-controlled pharmaceutical reactor, we provide safe, reliable, and certified solutions tailored to your process.
📩 Contact our technical team today to request a quote, share your specifications, or start your project discussion.